Engineering the World’s Smallest Cardiac Pacemaker
World’s smallest pacemaker is set to be a game changer for medical technology.
Written by Amanda Morris | Photos by Kim Le Mezo
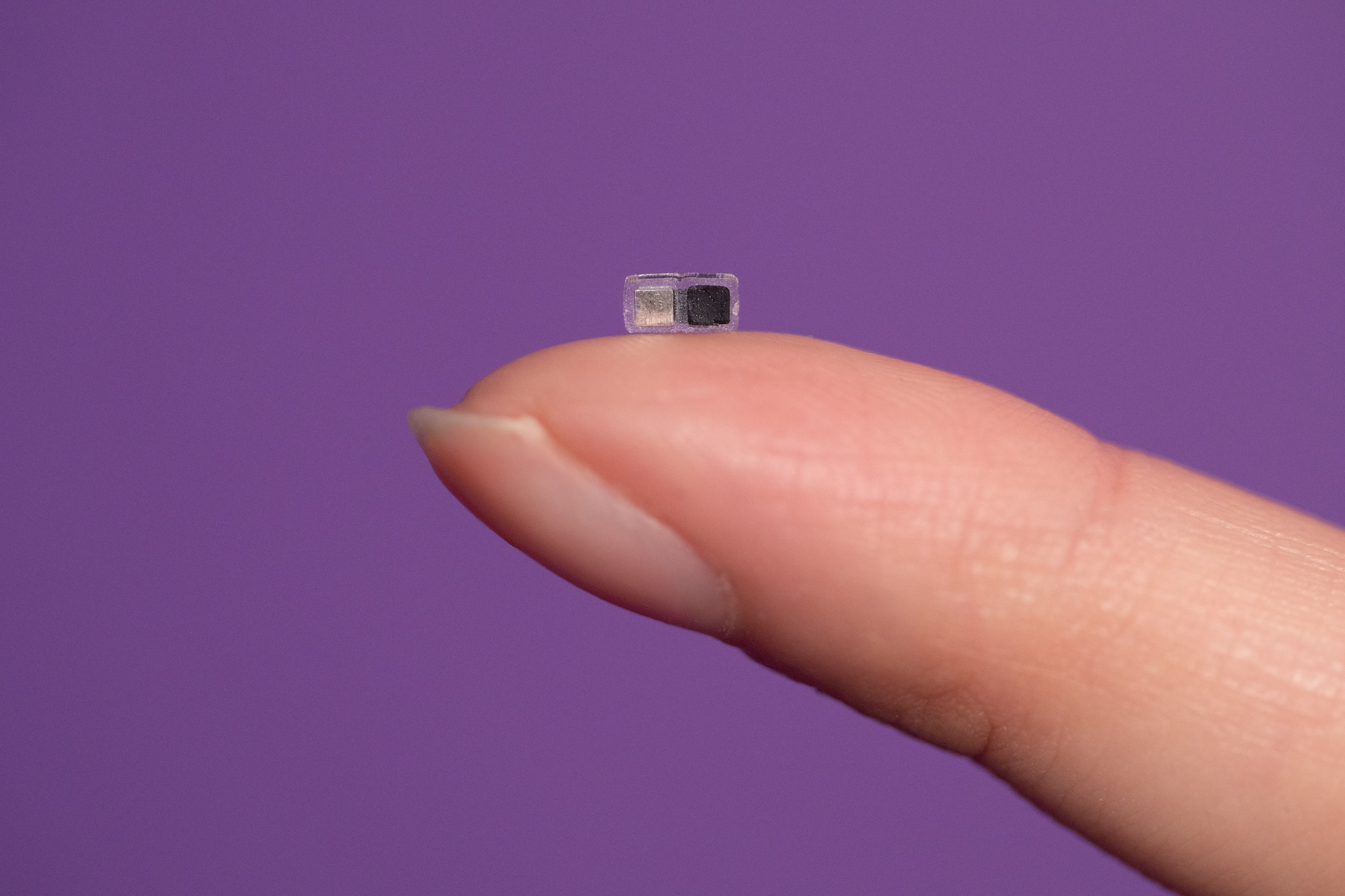
For patients with short-lived bradycardia, temporary pacemakers are an essential part of post-operative care. Conventional devices require invasive open-heart surgery or less-invasive endovascular surgery, both of which present a host of challenges including risks of infections, lacerations and perforations of the myocardium, and displacements of external power supplies and control systems.
To combat the risks and challenges associated with the use of pacemakers, Northwestern University engineers have developed a pacemaker so small that it can fit inside the tip of a syringe needle — and be non-invasively injected into the body, according to a new study published in Nature.
Smaller than a single grain of rice, the pacemaker is paired with a small, soft, flexible, wireless, wearable device that mounts onto a patient’s chest to control pacing. When the wearable device detects an irregular heartbeat, it automatically shines a light pulse to activate the pacemaker. These short pulses — which penetrate the patient’s skin, breastbone, and muscles — control the pacing.
Designed for patients who only need temporary pacing, the pacemaker simply dissolves after it’s no longer needed. All the pacemaker’s components are biocompatible, so they naturally dissolve into the body’s biofluids, by passing the need for surgical extraction. While it can work with hearts of all sizes, the pacemaker is particularly well suited to the tiny, fragile hearts of newborn babies with congenital heart defects.
“We have developed what is, to our knowledge, the world’s smallest pacemaker,” said John Rogers, PhD, the Louis Simpson and Kimberly Querrey Professor of Materials Science and Engineering, Biomedical Engineering, and Neurological Surgery, who led the device development. “There’s a crucial need for temporary pacemakers in the context of pediatric heart surgeries, and that’s a use case where size miniaturization is incredibly important. In terms of the device load on the body — the smaller, the better.”
“Our major motivation was children,” said Igor Efimov, PhD, professor of Medicine in the Division of Cardiology and in the McCormick School of Engineering, who co-led the study. “About 1 percent of children are born with congenital heart defects — regardless of whether they live in a low-resource or high-resource country. The good news is that these children only need temporary pacing after a surgery. In about seven days or so, most patients’ hearts will self-repair. But those seven days are absolutely critical. Now, we can place this tiny pacemaker on a child’s heart and stimulate it with a soft, gentle, wearable device. And no additional surgery is necessary to remove it.”
MEETING AN UNMET NEED
The creation of this new device was built on a previous collaboration between Rogers and Efimov that resulted in the first dissolvable device for temporary pacing. Many patients require temporary pacemakers after heart surgery — either while waiting for a permanent pacemaker or to help restore a normal heart rate during recovery. For the current standard of care, surgeons sew the pacemaker’s electrodes onto the heart muscle during surgery. Wires from the electrodes exit the front of a patient’s chest, where they connect to an external pacing box that delivers a current to control the heart’s rhythm. When the temporary pacemaker is no longer needed, physicians remove the pacemaker electrodes. Potential complications include infection, dislodgement, torn or damaged tissues, bleeding, and blood clots.
“Wires literally protrude from the body, attached to a pacemaker outside the body,” Efimov said. “When the pacemaker is no longer needed, a physician pulls it out. The wires can become enveloped in scar tissue. So when the wires are pulled out, that can potentially damage the heart muscle. That’s actually how Neil Armstrong died. He had a temporary pacemaker after a bypass surgery. When the wires were removed, he experienced internal bleeding.”
In response to this clinical need, Rogers, Efimov, and their teams developed their dissolvable pacemaker, which was introduced in Nature Biotechnology in 2021. The thin, flexible, lightweight device eliminated the need for bulky batteries and rigid hardware, including wires. Rogers’ lab had previously invented the concept of bioresorbable electronic medicine — electronics that provide a therapeutic benefit to the patient and then harmlessly dissolve in the body like absorbable sutures. By varying the composition and thickness of the materials in these devices, the team can control the precise number of days they remain functional before dissolving.
BODY FLUID-POWERED BATTERY
While the original quarter-size dissolvable pacemaker worked well in preclinical animal studies, cardiac surgeons sought to make the device smaller so it would be better suited to non-invasive implantation and for use in the smallest patients. However, the device was powered by near-field communication (NFC) protocols — the same technology used in smartphones for electronic payments and in radio-frequency identification (RFID) tags — which required a built-in antenna.

Even though the pacemaker is so tiny —
measuring just 1.8 millimeters in width,
3.5 millimeters in length, and 1 millimeter
in thickness — it still delivers as much
stimulation as a full-size pacemaker.
“Our original pacemaker worked well,” said Rogers, also the director of the Querrey Simpson Institute for Bioelectronics. “It was thin, flexible, and fully resorbable. But the size of its receiver antenna limited our ability to miniaturize it. Instead of using the radio frequency scheme for wireless control, we developed a light-based scheme for turning the pacemaker on and delivering stimulation pulses to the surface of the heart. This feature allowed us to dramatically reduce the size.”
To help further reduce the device’s size, the researchers also reimagined its power source. Instead of using near-field communication to supply power, the new, tiny pacemaker operates through the action of a galvanic cell, a type of simple battery that transforms chemical energy into electrical energy. Specifically, the pacemaker uses two different metals as electrodes to deliver electrical pulses to the heart. When in contact with surrounding biofluids, the electrodes form a battery. The resulting chemical reactions cause the electrical current to flow to stimulate the heart.
“When the pacemaker is implanted into the body, the surrounding biofluids act as the conducting electrolyte that electrically joins those two metal pads to form the battery,” Rogers said. “A very tiny light-activated switch on the opposite side from the battery allows us to turn the device from its ‘off’ state to an ‘on’ state upon delivery of light that passes through the patient’s body from the skin-mounted patch.”
PULSING WITH LIGHT
The team used an infrared wavelength of light that penetrates deeply and safely into the body. If the patient’s heart rate drops below a certain rate, the wearable device detects the event and automatically activates a light-emitting diode. The light then flashes on and off at a rate that corresponds to the normal heart rate.
“Infrared light penetrates very well through the body,” Efimov said. “If you put a flashlight against your palm, you will see the light glow through the other side of your hand. It turns out that our bodies are great conductors of light.”
Even though the pacemaker is so tiny — measuring just 1.8 millimeters in width, 3.5 millimeters in length, and 1 millimeter in thickness — it still delivers as much stimulation as a full-size pacemaker.
“The heart requires a tiny amount of electrical stimulation,” Rogers said. “By minimizing the size, we dramatically simplify the implantation procedures, we reduce trauma and risk to the patient, and, with the dissolvable nature of the device, we eliminate any need for secondary surgical extraction procedures.”
MORE SOPHISTICATED SYNCHRONIZATION
Because the devices are so tiny, physicians could distribute collections of them across the heart. A different color of light could illuminate to independently control a specific pacemaker. Use of multiple pacemakers in this manner enables more sophisticated synchronization compared to traditional pacing. In special cases, different areas of the heart can be paced at different rhythms — for example, to terminate arrhythmias.
“We can deploy a number of such small pacemakers onto the outside of the heart and control each one,” Efimov said. “Then we can achieve improved synchronized functional care. We also could incorporate our pacemakers into other medical devices, like heart valve replacements, which can cause heart block.”
“Because it’s so small, this pacemaker can be integrated with almost any kind of implantable device,” Rogers said. “We also demonstrated integration of collections of these devices across the frameworks that serve as transcatheter aortic valve replacements. Here, the tiny pacemakers can be activated as necessary to address complications that can occur during a patient’s recovery process. So that’s just one example of how we can enhance traditional implants by providing more functional stimulation.”
The technology’s versatility opens a broad range of other possibilities for use in bioelectronic medicines, including helping nerves and bones heal, treating wounds, and blocking pain. And while the device has only been studied in use on animals, Rogers foresees it being used in clinical trials in about five years. This would be the first resorbable electronic device ever used in a clinical setting, and Rogers and his team have much more work ahead to ensure its safety before it can be used in live patients.
“All our evaluations confirm that the technology works very well,” Rogers said. “And our team is working aggressively to have this implantable, resorbable device used as a life-saving tool for critically ill patients of all sizes — after passing through the regulatory process — as soon as possible.”
EXPLORE MORE
Get an in-depth look at how Northwestern engineers and Feinberg investigators came together to develop this innovative solution to meet a need for patients.