Outsmarting a Trickster
Feinberg scientists have discovered how herpesviruses invade the body — paving the way for vaccine development.
by Melissa Rohman
photos by Gr8y Productions

Greg Smith, PhD, and his laboratory have made a breakthrough discovery about the herpesvirus.
Greg Smith, PhD, was a graduate student at the University of Pennsylvania studying food-borne bacterial pathogens when he became fascinated with the ingenuity of herpesviruses and how they manage to invade the immune system.
“I was really amazed at how pathogens are basically the best cell biologists there are,” says Smith, who is now a professor of Microbiology-Immunology at Feinberg. “We can learn a lot about ourselves by studying what they know about us. From that point on, I was off and running, trying to learn about how these viruses do the amazing tricks that they do.”
Smith’s 20-year pursuit of the herpesvirus recently crossed a monumental marker with his lab’s discovery that herpesviruses perform a devious maneuver to effectively invade and hide within the nervous system for life.
The understanding of this trickery, described in a recent study published in Nature, lays the groundwork for the development of new vaccines that can prevent both herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) from reaching the nervous system in the first place, Smith says.
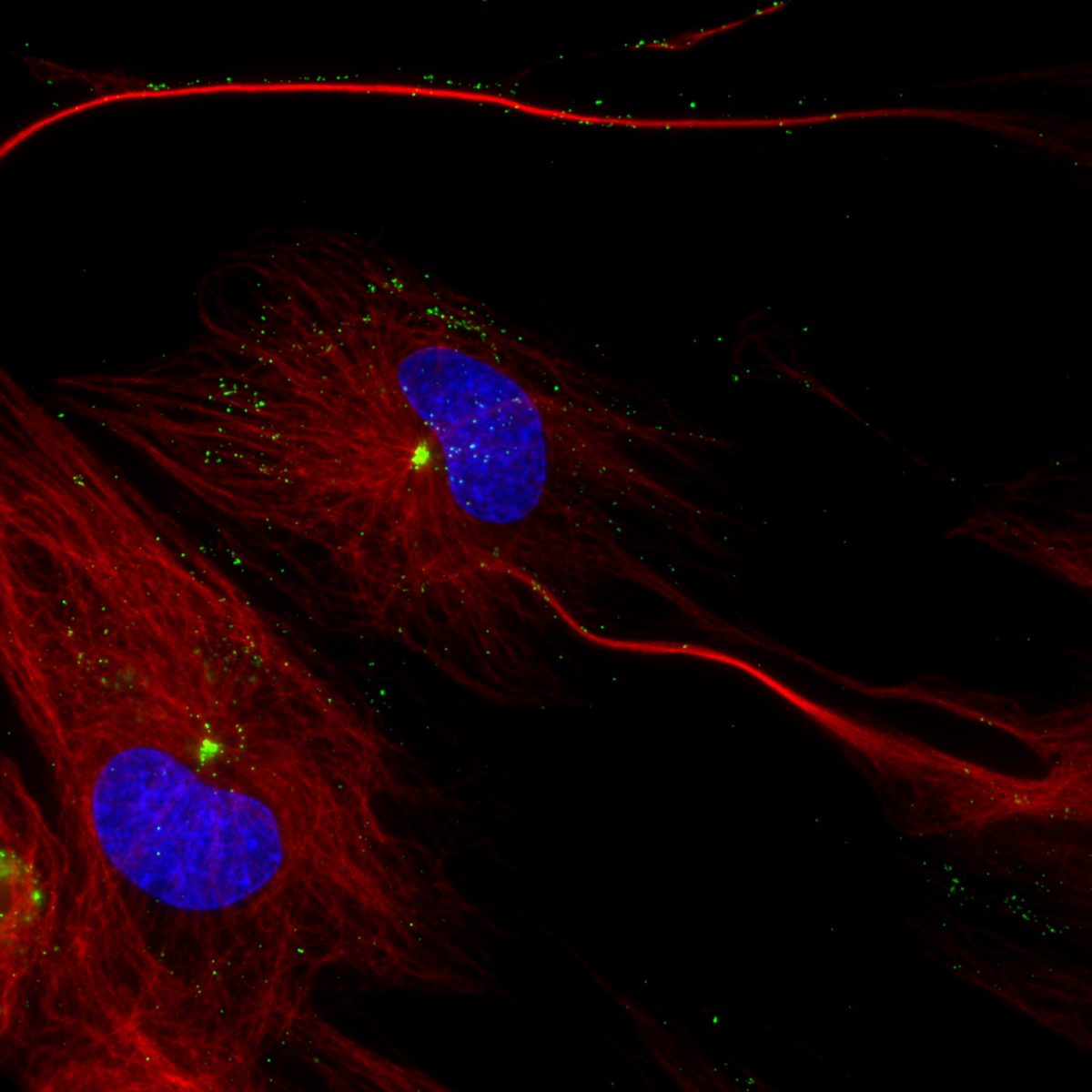
centrosome.
PERSISTENT PATHOGENS
Herpesviruses are an ancient family of viruses that cause lifelong infection. While they are treatable, there is no cure. Herpesviruses can infect humans and other mammals, but over time, individual herpesviruses have evolved to infect just one species.
Of the more than 100 known herpesviruses, there are several known to infect the human nervous system and manifest disease, an evolutionary triumph that Smith has been investigating in his laboratory for decades.
HSV-1 and its close sibling HSV-2 are the most well-known herpesviruses that infect humans. According to the World Health Organization, more than half of the world’s population are carriers of HSV-1. The virus is transmitted through oral contact, and many who become infected will experience as little as a cold sore. But the manifestation of HSV-1 infection, like any infection, can vary widely. For some, HSV-1 can be life-threatening, causing blindness or severe encephalitis, or inflammation of the brain.
Meanwhile, HSV-2 is sexually transmitted, but can also be passed from mother to newborn during birth as neonatal herpes. The virus can cause brain damage or organ failure for the baby if left untreated.
Smith’s laboratory studies two neuroinvasive herpesviruses: HSV-1 and pseudorabies virus, a veterinary herpesvirus that infects pigs. His laboratory uses live-cell fluorescence microscopy, molecular genetics, and neuronal cell biology to study the molecular mechanisms behind neuroinvasion and pathogenesis.
KNOW YOUR ENEMY
The exact molecular mechanisms that herpesviruses employ to invade the nervous system have remained unknown — until now.
By performing live imaging of cell cultures manually infected with HSV-1 and pseudorabies virus, Smith’s team found that herpesviruses hijack a protein from epithelial cells to help it travel into the nervous system, a phenomenon unlike any other known virus. This allows the virus to invade the nervous system with incredibly high frequency through a process Smith’s team has termed “assimilation.”
If you want to make a vaccine, if you want to fight your enemy, you need to know and understand your enemy.
GREG SMITH, PHD
“The protein is no longer an active participant of your natural cell biology because it’s defected to the other team and has become an active component of the virus. It’s been ‘assimilated’ by the virus to be part of it in a productive way,” Smith says.
Once the virus moves past the outer membrane of mucosal epithelial cells, it must navigate its way through the cell to reach the nucleus to successfully replicate. Like other viruses, herpesviruses journey through the cell by traveling along highways called microtubules, which originate from the cell’s centrosome, and use two proteins as their motors: dynein and kinesin.
But dynein and kinesin travel in opposing directions — one travels towards the nucleus while the other diverts away from it. Many common viruses use both dynein and kinesin to travel along microtubules to eventually reach the nucleus. But for herpesviruses, traveling down a neuron is a much longer journey.
“It’s about an eight-hour marathon run for the virus to get into our nervous system,” Smith says.
The virus first uses the dynein motor to travel through the cell as far as it can until it reaches the centrosome, where it can’t travel any further. Determined to reach the nucleus, however, the virus pulls its trick.
It reaches into its stores and pulls out a kinesin stolen from the mucosal epithelial cells. Full speed ahead, the virus uses the foreign kinesin to travel in the opposing direction and reach its destination: the nucleus.
“We have described a new principle in virology: viral assimilation — the repurposing of a cellular protein as an essential virion component that drives subsequent rounds of infection,” says Caitlin Pegg, a student in the Driskill Graduate Program in Life Sciences (DGP) and lead author of the Nature study. The herpesviruses steal the kinesin from epithelial cells, carry it with them into the nervous system, and then use it precisely at the moment it is needed.
Assimilation is an evolutionarily triumph for herpesviruses to gain new abilities without having to undergo extensive genetic changes, according to Smith, and may be relevant to many other viruses, such as HIV and SARS-CoV-2.
More importantly, understanding the robust molecular mechanisms responsible for the neuroinvasive nature of herpesviruses is key to developing new vaccines to treat herpesviruses.
According to Smith, a potential vaccine that inhibits assimilation altogether may be an effective therapeutic approach in preventing herpesviruses from infecting the nervous system in the first place. “If you want to make a vaccine, if you want to fight your enemy, you need to know and understand your enemy,” he says.

From left to right: Jeffrey Savas, PhD, Derek Walsh, PhD, Greg Smith, PhD, graduate student Caitlin Pegg, Vladimir Jovasevic, PhD, research assistant professor in the Department of Pharmacology, and graduate student DongHo Kim, all collaborate in Smith’s lab.
PREPARING THE FRONT LINES
Currently, there is only one available therapy known to effectively treat active herpes infections. The antiviral drug, called Acyclovir, prevents herpesviruses from actively replicating. However, it’s unable to eliminate a virus after it has already established a latent infection in the nervous system.
“The problem is these viruses can, on occasion, cause severe disease, including being lethal. It’s a question of rolling that dice — just because you survived it like most people doesn’t mean that somebody else isn’t going to get a life-threatening infection,” Smith says.
So how can severe infection from herpesviruses be prevented? The answer, according to Smith, is to create a live attenuated vaccine that engages the entire immune system, preparing it to immediately recognize and fend off herpesviruses before they’re ever able reach the nervous system.
“Herpes is not playing around. It’s a virus on a mission that knows what it needs to do, so we’ve got to get the full immune arsenals out there to stop it,” Smith says.
Live-attenuated vaccines use weakened forms of a virus to create a long-lasting immune response. This is currently the standard for measles and influenza vaccines, and was the type of vaccine that eliminated smallpox. Smith says this novel non-neuroinvasive herpesvirus vaccine would have to be administered to people before they’re exposed to the virus, which most likely means getting the vaccine in early childhood. This would ensure the immune system is prepared and ready to attack the virus at any given moment, reducing the risks of severe disease and complications from life-long infections. Now, Smith and his team are working to make this a reality.
In partnership with Northwestern University, Smith and two of his colleagues founded Thyreos, Inc. to produce non-neuroinvasive herpesvirus vaccines. “By discovering how these viruses invade the nervous system, we can now make innocuous virus strains that specifically lack this ability. And in so doing, these viruses can only replicate in mucosal tissues, much like a common cold virus,” Smith says. The active replication of these vaccines in the mucosa elicits a potent response from the immune system, and will hopefully achieve what no other vaccine has done before: protect us from herpesvirus infections.
Other Northwestern contributors to the study are Sofia Zaichick, PhD; and the laboratories of Jeffrey Savas, PhD, assistant professor in the Ken and Ruth Davee Department of Neurology Division of Behavioral Neurology; and Derek Walsh, PhD, professor of Microbiology-Immunology. The laboratories of Duncan Wilson, PhD (Albert Einstein College of Medicine) and Patricia Sollars, PhD, and Gary Pickard, PhD (University of Nebraska-Lincoln), also contributed to the study.
To learn more, listen to our Breakthroughs podcast with Smith.