Next-Level Wearable Tech
Northwestern scientists are developing a wide array of sensing and therapeutic devices that can be easily integrated on and within our bodies.
by Emily Ayshford

A sticker that diagnoses cystic fibrosis. A wireless sensor that monitors blood flow in the brain. A potentially game-changing implantable device that relieves pain on demand — without the use of drugs. Each of these wearable and implantable technologies is a leap of innovation on its own, but it’s only a fraction of what John Rogers, PhD, has achieved in the past two years.
Using Wearables to Change AFib Treatment Guidelines

Early in his career, cardiac electrophysiologist Rod Passman, MD, had a patient with an abnormal heartbeat — a condition called atrial fibrillation (AFib) – that was well controlled with medications. Guidelines then (and now) say that patients with AFib should be on blood thinners continuously because they are at increased risk for stroke, regardless of how much time patients spend in AFib.
But in this case, the patient suffered from an intracranial bleed — worsened by the blood thinner. The bleed caused lasting physical and mental damage. “It ruined his life,” says Passman, director of the Center for Arrhythmia Research and the Jules J. Reingold Professor of Electrophysiology in the Department of Medicine. “It was a disaster from everyone’s perspective.”
Passman considered whether everyone who had abnormal heart rhythms needed to be on such medication, especially when newer blood thinners were put on the market that only took an hour or two to thin the blood and technologies that could continuously and remotely monitor the heart were becoming available.
Through a $10,000 philanthropic gift from a donor, Passman was able to work with a company that developed an implantable cardiac monitor to develop a pilot study which was published in Journal of Cardiac Electrophysiology. The idea was to only put patients on blood thinners for a few weeks only in response to a prolonged episode of AFib. And while the pilot study showed the concept was feasible, the chip itself was expensive and therefore not scalable to the tens of millions of people around the world who suffer from this disorder.
When wearable devices like the Apple Watch came to the market, Passman saw a chance to monitor heart rhythms in a wider range of patients and create a new era of personalized medicine for stroke prevention. While it is estimated that between 2.5 and 5 million Americans are living with AFib today, that number is estimated to grow to 12.1 million by 2030.
Now, Northwestern and Johns Hopkins University have been awarded a $37 million grant from the National Heart, Lung, and Blood Institute to fund a seven-year trial, called the Rhythm Evaluation for AntiCoagulaTion (REACT-AF) trial that will incorporate the use of an app on Apple Watch to monitor AFib to attempt to reduce patients’ continuous and lifelong reliance on blood-thinning medication.
In the trial, patients will wear an Apple Watch, which can monitor heart activity and notify patients when they’re entering an episode. When notified, patients will take blood-thinning medication for a few weeks during the high-risk window for stroke and can discontinue if they do not have another episode. The trial will begin enrolling patients in the spring of 2023. Partnering institutions include Johns Hopkins, Stanford University, and University of California at San Francisco.
Passman hopes that if the study shows this strategy is safe, it will change the way the condition is managed. “To have a tragic patient experience and use that to develop a pilot study and ultimately take the idea to clinical trial has been an amazingly rewarding experience,” Passman says. “As clinicians, we see gaps in treatment every day. This shows there is always room in medicine for questioning and challenging the dogma.”
Listen to the Rod Passman Breakthroughs podcast
With an engineer’s mindset and input from clinicians and investigators across the medical spectrum at Feinberg, Rogers and his team take complex hospital-room apparatuses — clunky machines, sensors strung with wires — and collapse them into tiny flexible devices that can be placed wire-free on the skin. For implantable devices, they are also re-imagining how such devices can interact with vital organs as needed — whether to pace a heart or numb nerves — then dissolve harmlessly once their job is finished.
As the Louis Simpson and Kimberly Querrey Professor of Materials Science and Engineering, Biomedical Engineering, Dermatology, and Neurological Surgery, Rogers has been innovating and refining these sorts of devices for years, ever since he developed the first-ever stretchable transistor in 2006. When he arrived at Northwestern from the University of Illinois Urbana-Champaign in 2016, he was already famous for his innovations in the field but wanted his group “more intimately embedded in a robust, broad medical community,” he says.
“Our hypothesis was that we would be able to further accelerate our translational research if we worked closely with a research-intensive medical school and a vibrant broader medical community — things have turned better than we could have possibly imagined,” he says.
Helping the most vulnerable patients: soft sensors
Rogers’ wearable sensors have flipped the idea of planar, rigid electronics that dominate medical technology. His team’s devices are soft, stretchy, and flexible, adhering gently to the skin and moving easily with the body.
In 2019, his team developed these soft sensors to monitor babies in the neonatal intensive care unit, measuring their vital signs without the need for wires. Developed in collaboration with Amy Paller, MD, the chair and Walter J. Hamlin Professor of Dermatology, and other collaborators at Ann & Robert H. Lurie Children’s Hospital of Chicago, the sensors were a breakthrough for premature babies and their parents, who often dealt with tangles of wires that prevented cuddling and physical bonding. The study was published in the journal Science. (Listen to Rogers and Paller talk about tech in the NICU on the Breakthroughs podcast.)
“If you consider what patient population would be most positively impacted by a soft, wireless medical technology, you think about vulnerable patients, where premature babies come in at the top of the list,” Rogers says. “As a result, we prioritized efforts to gear our technology around the needs of those patients, given the profound potential benefits.”
That sort of motivation has continued to push innovation in his lab. More recently, the team developed a novel wireless device about the size of a Band-Aid that improves real-time monitoring of blood flow and oxygenation in the brain for neonatal and pediatric patients. This sort of monitoring helps ensure proper neurodevelopment, especially for those in intensive care units who require constant care and observation. The work was published in Proceedings of the National Academy of Sciences (PNAS).
They’ve also developed new ways to test newborns for potential diseases. All newborns are screened for cystic fibrosis, the most-common life-shortening genetic disease. Currently, the screening involves blood through a heel prick, and if that screen is abnormal, pediatricians order a sweat test. During the sweat test, the baby must wear a hard, wrist-strapped device for up to 30 minutes to collect sweat, separately analyzed using a benchtop apparatus, all conducted by trained medical personnel.
Rogers and his collaborators, including Susanna McColley, MD, professor of Pediatrics in the Division of Pulmonary and Sleep Medicine, a cystic fibrosis expert, and a Lurie Children’s and Northwestern Medicine pediatric pulmonologist, developed a novel skin-mounted sticker that captures sweat in microfluidic channels that pass to microscale reservoirs that change color to provide an accurate, easy-to-read diagnosis of cystic fibrosis within minutes. Unlike standard testing protocols, the sticker is easy to use, comfortable on the skin and low in cost, making it suitable for testing in remote or resource constrained areas of the world. The study was published in Science Translational Medicine.
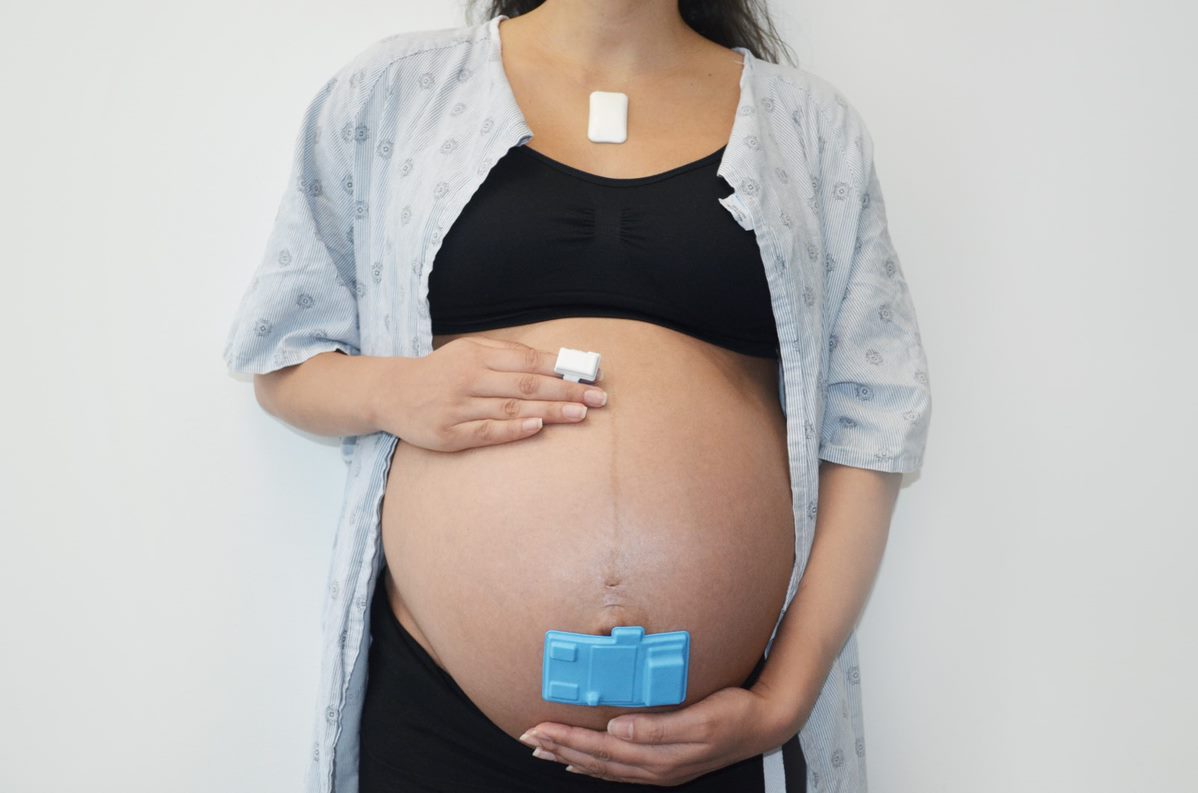
Funding from the Gates Foundation led the researchers to also consider the entire maternal-fetal cycle, and soon the team had tackled the cumbersome fetal-monitoring belts that pregnant women must wear in the hospital and during labor. With Rogers’ system, pregnant women wear three small, soft, flexible wireless sensors that measure the mother’s and baby’s vital signs simultaneously, as well as provide new data, including information about the mother’s physical movements and laboring positions, that is not collected with current technology. The sensors were developed with Rogers’ frequent collaborator Shuai “Steve” Xu, MD, MSc, the Ruth K. Freinkel, MD, Professor and assistant professor of Dermatology and of Pediatrics in the Division of Dermatology. The work, which was published in the Proceedings of the National Academy of Sciences, included data not only from Prentice Women’s Hospital, but also from nearly 500 women throughout the intrapartum period in health clinics in Zambia, demonstrating the ability to operate cost-effectively in environments with limited hospital infrastructure. Xu and Rogers also collaborated on a wearable sensor that tracks children’s suffering with eczema, and adults with itch symptoms, publishing the study in Science Advances.
The possibilities with these wearable sensors are seemingly endless. “Now that we have a foothold in this area, we’re developing other kinds of sensors we can drop into these same basic soft, wireless platforms,” he says. “We are aiming not only to reproduce the measurements that form the standard of care today, but also to pioneer new classes of sensors, all under the umbrella of advanced techniques in data analytics. There’s a tremendous amount of opportunity in that direction.”
A temporary, dissolving pacemaker
As Rogers and his team had conversations with Feinberg investigators, they began to understand various clinical uses of devices that are implanted into the body to perform a temporary function that addresses a transient patient need, and then are surgically extracted. Cardiac surgeons, for example, asked if his team could develop an alternative to the wired, temporary pacemakers used with patients during recovery following certain types of cardiac surgeries.
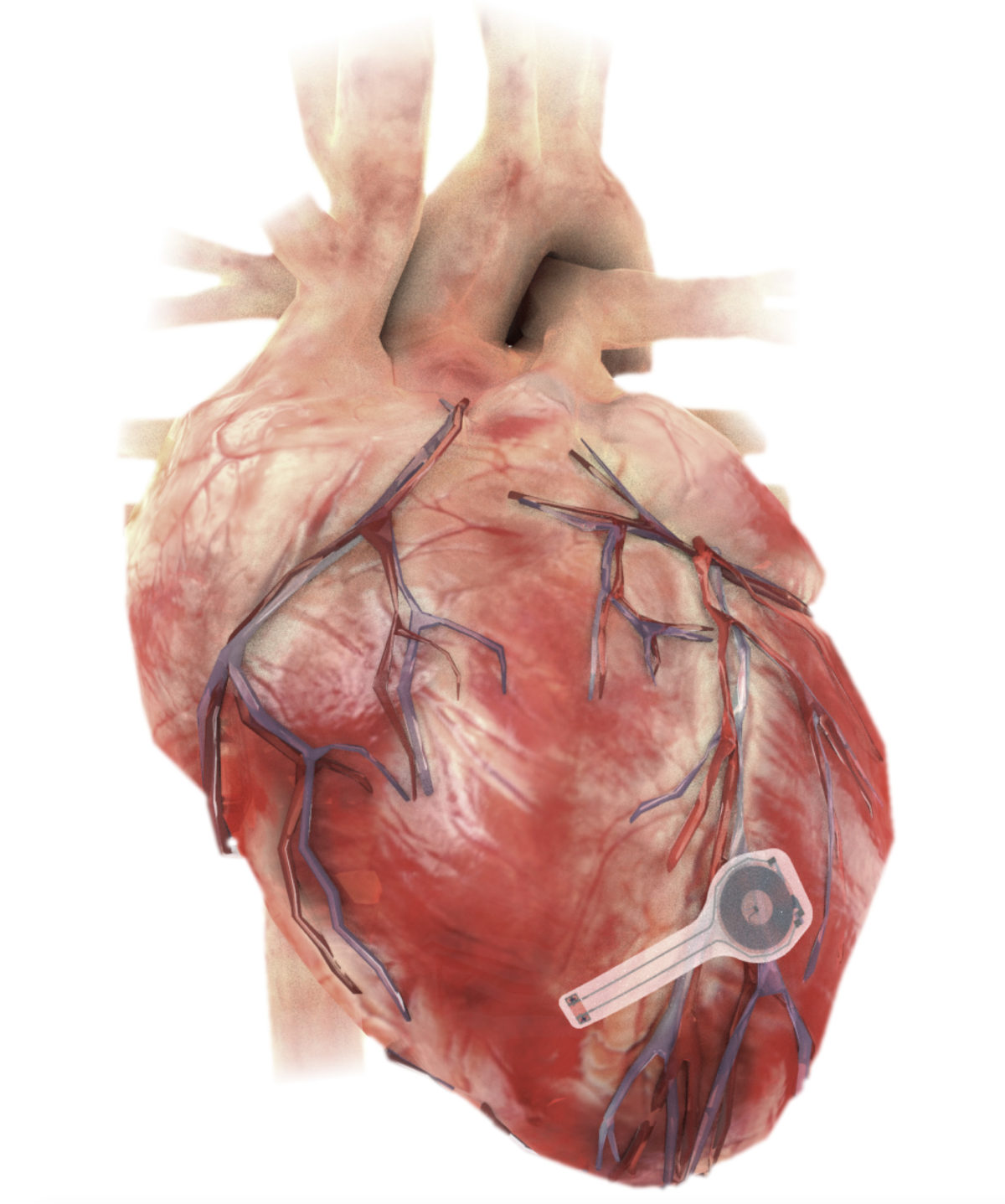
A skin-mounted and implanted device monitors body temperature, oxygen levels, respiration, muscle tone, physical activity, and the heart’s electrical activity.
The idea for a wireless, bioresorbable electronics technology had intrigued his team for years. In fact, some years ago they developed a collection of materials and manufacturing techniques for integrated circuits that can dissolve in water, in the context of a Defense Advanced Research Projects Agency funded project in transient electronics for military purposes. They were interested in possibilities for adapting that technology for patient care. In 2022, they introduced an implantable, wireless pacemaker that is integrated into a coordinated network of four soft, flexible, wireless, wearable sensors and control units placed around the upper body. The work was published in Science.
This wireless network of skin-mounted and implanted devices monitors body temperature, oxygen levels, respiration, muscle tone, physical activity, and the heart’s electrical activity. The system was developed in a collaboration with Rishi Arora, MD, professor, and Anna Pfenniger, MD, PhD, assistant professor, of Medicine in the Division of Cardiology, and Bradley Knight, MD, the Chester C. and Deborah M. Cooley Distinguished Professor of Cardiology.
The system then uses algorithms to analyze this combined activity in order to autonomously detect abnormal cardiac rhythms and decide when to pace the heart and at what rate. All this information is streamed to a smartphone or tablet, so physicians can remotely monitor their patients.
“Joining these two areas — wireless sensors on the skin and bioresorbable implants in the body — could potentially allow patients to be released from the hospital earlier following a cardiac surgery,” Rogers says.
An implantable device to treat pain
Perhaps the most exciting of Rogers’s recent work, published in Science, is a new dissolving implantable device that relieves pain on demand without drugs. The biocompatible, water-soluble device works by softly wrapping around nerves to deliver precise, targeted cooling, which numbs nerves and blocks pain signals to the brain.
An external pump enables the user to remotely activate the device and then increase or decrease its cooling intensity, precisely controlled through feedback from an integrated digital temperature sensor at the site of the nerve. After the device is no longer needed, it naturally absorbs into the body — bypassing the need for surgical extraction.
Clinicians believe the device will be most valuable for patients who undergo routine surgeries or even amputations that commonly require post-operative medications. Surgeons could implant the device during the procedure to help manage the patient’s post-operative pain.
“Although opioids are extremely effective, they also are extremely addictive,” Rogers says. “As engineers, we were motivated by the idea of treating pain without drugs — in ways that can be turned on and off instantly, with user control over the intensity of relief.”
Creating a closed loop of sensing and therapy
As for what devices Rogers will develop next — the future is wide open. His team seems to be perpetually in a state of being simultaneously maxed out with existing projects and looking for new ones, working with Feinberg collaborators to identify the most important unmet needs that could be addressed with new technology.
They’re also regularly innovating on their wireless sensor platforms, adding in biochemical sensors that could continuously track cortisol levels or inflammatory markers, as examples. They are also hoping to use machine learning to discover new insights in the vast streams of data that their devices record.
“An important goal is to continue to work on systems that combine both sensing and therapeutics — pulling together multiple different technologies that are all wirelessly synchronized to both sense and treat conditions in a closed loop operation,” Rogers says.
— Amanda Morris, Kristin Samuelson, and Melissa Rohman contributed to this story.