A New Path
Northwestern scientists tackle a common — yet elusive — type of heart disease with novel approaches and a new urgency.
by Melissa Rohman
While completing his cardiology fellowships in the mid 2000s, Sanjiv Shah, ’00 MD, often lamented the lack of specialized research programs for an increasingly recognized type of heart disease — heart failure with preserved ejection fraction (HFpEF), which is strikingly different from the traditional heart failure condition driven by a weak heart muscle. Moreover, he was struck by the shortage of medical attention provided to these patients.

“I was thinking to myself, ‘No one is really focusing on the care of these patients.’ Clinical trial enrollment was dismal,” says Shah, who is the Neil J. Stone, MD, Professor of Medicine in the Division of Cardiology and director of research at the Bluhm Cardiovascular Institute.
Formerly known as diastolic heart failure, but now appreciated as a much more complex perturbation effectively limiting the performance of the heart despite an apparent intact muscle, HFpEF occurs when the heart is unable to relax and fill with enough blood during each heartbeat. To compensate, pressure increases in the heart so it can properly fill with blood and support heart performance. Typically, this mechanism fails. Consequently, fluid builds in the lungs, leading to shortness of breath, fatigue, irregular heartbeat, and, ultimately, heart failure.
Of the estimated six million patients in the U.S. living with heart failure, over half have HFpEF. Currently, HFpEF accounts for most heart failure-related hospitalizations in the U.S. for people over the age of 65.
Yet, HFpEF has been historically misunderstood, resulting in misdiagnosis and limited treatments. When he was training, Shah says he remembers many in the field thought HFpEF simply equated to a stiff heart muscle that couldn’t relax properly. While this was in part true, in reality, there was more to the condition.
“When these patients exercise, their heart typically doesn’t squeeze as well as it should, so it’s not simply a problem of a stiff heart unable to fill. And HFpEF affects several other organs besides the heart,” Shah says. “It involves the skeletal muscles, the lungs, the kidney, the adipose tissue, even the liver.”

This revelation prompted Shah to establish the world’s first dedicated HFpEF clinical program, which found a home at Northwestern in 2007. The program aims to improve HFpEF prevention, diagnosis, treatment, and prognosis through pioneering research and expert clinical care.
“When I first started at Northwestern, I thought that this syndrome is much more complex than most realized, and I think anyone who takes care of a lot of these patients recognizes that HFpEF is heterogeneous. Therefore, one of my main goals when starting the HFpEF program at Northwestern was to improve classification of HFpEF subtypes in order to guide treatment approaches,” Shah says.
Now, Shah and other Feinberg investigators including Sadiya Khan, ’09 MD, ’14 MSc, ’10, ’12 GME, assistant professor of Medicine in the Division of Cardiology and of Preventive Medicine in the Division of Epidemiology, are continuing to study the underlying mechanisms of HFpEF with the hope of better preventing the disease and identifying novel therapeutic targets and strategies.
“It’s really exciting to say we’re getting more and more precise, that the work we’re doing is really seeming to make a benefit, and that we’re really getting close, hopefully to not just one class of drugs for this patient population, but a whole host of drugs and devices to improve this disorder,” Shah says.
Investigating HFpEF
There are currently no simple diagnostic tests for HFpEF, leaving providers to rely on interpreting patient signs and symptoms or cardiac imaging results.
The lack of clear definitions for HFpEF subtypes has also contributed to confusion and misdiagnosis and, therefore, not all HFpEF patients respond favorably to the same treatment. A one-size-fits-all approach has been tried numerous times in HFpEF clinical trials with little success. Recently, a few proven treatments tested in trials co-led by Shah and colleagues have been FDA-approved and are now available; however, even with these treatments, patients with HFpEF still have high morbidity and mortality.
But the future looks bright. In just the last 15 years, Northwestern investigators have discovered more about the underlying biological basis of HFpEF than ever before. For example, they have provided evidence, published in Circulation, that risk factors such as obesity, metabolic stress, hypertension, and physical inactivity are associated with a distinct protein “signature” in the blood that results in abnormalities in heart function in HFpEF patients. In addition, Shah and colleagues pioneered the use of machine-learning techniques to classify complex clinical syndromes such as HFpEF; in 2015, they published the first study on a data-driven technique they called “phenomapping” to classify HFpEF patients into novel, distinct subtype, also published in Circulation.
Most recently, Shah’s team discovered that some patients with HFpEF might benefit from an intentional communication between the upper chambers of the heart, or, an atrial shunt, according to findings published in The Lancet. The novel, minimally invasive device, which is inserted through a catheter, could potentially lower pressure in the heart’s left atrium and reduce HFpEF symptoms.
Feinberg investigators have also made significant contributions to the advancement of HFpEF treatments and identifying at-risk patient groups. In a study published in Nature Medicine, a team of investigators including Shah and Khan found that dapagliflozin — a drug commonly used to treat type 2 diabetes — improved symptoms and physical limitations in patients with HFpEF.
It’s really phenomenal. Finally, we are able to tell patients we have a drug that will make them feel better, do more, and stay out of the hospital.
Sanjiv Shah, ’00 MD
“It’s really phenomenal. Finally, we are able to tell patients we have a drug that will make them feel better, do more, and stay out of the hospital,” Shah says.
But the battle isn’t over, according to Shah. In order to pinpoint poorly differentiated diseases such as HFpEF and identify effective treatments, Shah said the field needs to move away from large clinical trials and instead leverage clinical data that’s readily available.
“I think that’s really the goal of precision medicine,” Shah says. “It’s not perfect and we have a long way to go, but we really have to think about leveraging all the data we have now to come to a future where we’re more precisely identifying and defining these clinical syndromes.”
Improving Diagnosis and Treatment
In addition to the work in Shah’s clinic, Feinberg investigators were recently awarded an $18.1 million grant from the National Institutes of Health (NIH) to study the underlying pathophysiology of HFpEF. The grant is part of the NIH HeartShare program, a multi-institutional research effort with the goal of characterizing the mechanisms driving HFpEF and identifying new therapies.
Shah leads the HeartShare Data Translation Center at Northwestern, the central hub of the program. Shah’s center coordinates all aspects of the HeartShare program, including the collection of data, images, and molecular data on thousands of patients from numerous previously conducted studies; the center is also directing a prospective study of 1,000 patients recruited at all six HeartShare program sites.
Investigators in the program will perform exercise testing, imaging of the heart and other organs, and phenotyping of blood and other tissues. Machine learning simultaneously applied to electronic health records, echocardiograms (heart ultrasounds), heart MRIs, CT scans, EKGs, and molecular analyses from the blood will enable improved determination of heart failure subtypes, with the goal of unraveling of novel biological mechanisms underlying HFpEF.
“The HeartShare grant from the NIH is a phenomenal opportunity to continue our research to improve the classification of the heterogeneous HFpEF syndrome, understand the biological basis of HFpEF phenotypes, and set the stage for precision medicine clinical trials for HFpEF,” says Shah, who is also director of the Institute for Artificial Intelligence in Medicine’s Center for Deep Phenotyping and Precision Therapeutics.
Northwestern will also be home to one of HeartShare’s six clinical centers. Khan will lead the center, along with Laura Rasmussen-Torvik, PhD, chief of Epidemiology in the Department of Preventive Medicine. The center at Northwestern intends on leading the six sites in recruitment and enrollment of patients with HFpEF.

“HeartShare is a unique program that will catalyze the discovery of novel prevention and therapeutic options for patients living with or at risk for HFpEF,” Khan says. “It is an incredible honor to be part of the program, which will redefine the paradigm for defining HFpEF and fundamentally shift our approach to caring for these patients.”
The time is right for a paradigm shift, agrees Clyde Yancy, MD, MSc, the Magerstadt Professor and vice dean for Diversity and Inclusion, chief of Cardiology in the Department of Medicine and a professor of Medical Social Sciences.
“Collectively, the study of heart failure has come far; we have multiple new effective therapies, updated national treatment algorithms, and new hope to restore quality of life and longevity in patients with traditional heart failure. But we have not sufficiently addressed a critical need: HFpEF,” he says. “Uniquely here at Northwestern, we have the talent and now the resources to allow further study of the pathophysiology and future treatment of this challenging iteration of heart failure.”
To learn more about HFpEF, listen to our recent Breakthroughs podcast with Shah.
The Role of Exercise
The Lancet study that found patients with HFpEF could benefit from an atrial shunt also offers new insight into the role exercise plays in understanding, diagnosing, and treating this type of heart failure.
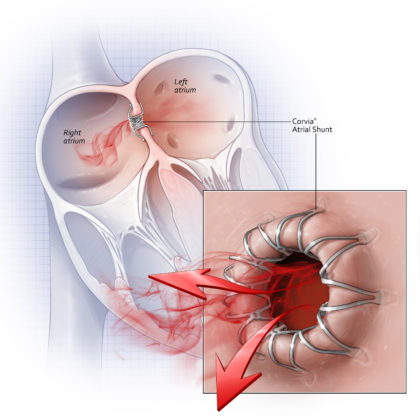
“While the overall trial was neutral, in our pre-specified subgroup analyses we found that what happens in
the heart and lungs during exercise is of prime importance in this type of heart failure,” Shah says. “The normal response to exercise is relaxation of the blood vessels in the lungs. Patients with HFpEF who are able to relax the blood vessels in their lungs appear to do well with the device, whereas those whose blood vessels can’t relax appear to do worse when an atrial shunt is implanted.”
While cardiovascular conditions such as coronary artery disease are routinely diagnosed with exercise testing, clinical assessment for HFpEF is done at rest — something that Shah says he hopes will change following this trial.
“This has potential to change the way we evaluate patients with this condition and should guide how future clinical trials are conducted and the criteria for enrollment,” Shah says. “If future trials validate what we found, the potential is enormous. This subgroup comprises two-thirds of people with this type of heart failure — that is 2 million people who could benefit from this innovative therapy.”
The Corvia REDUCE LAP-HF II pivotal trial is the largest device study ever conducted in HFpEF and the first pivotal trial of interatrial shunts completed. The results were also presented at the Cardiology Research Foundation (CRF)’s Technology and Heart Failure Therapeutics meeting earlier this year. Funding for the study was provided by Corvia Medical.