Portrait of a Tumor
A new DNA methylation profiling tool that ensures brain tumors are accurately diagnosed is available for patients of Northwestern Medicine.
by Emily Ayshford
Photography by Laura Brown

Using a new diagnostic technology now available at Northwestern Medicine, Craig Horbinski, MD, PhD, is able to identify a tumor’s unique methylation fingerprint.
When Bonnie Fiorito started having bad headaches in October 2021, she investigated possible culprits. Her first stop was a visit with an optometrist, who told her she needed glasses, badly. And when she had an awful headache again after wearing glasses, she thought her brain might just be adjusting to finally seeing clearly.
As a healthy 31-year-old who enjoyed running, she’d never had any major health problems, so she dealt with the headaches and continued working as a nanny. She even got engaged on December 18. But after a series of massive headaches over Christmas that same year, she finally went to the emergency room, where a CT scan and MRI showed a mass in her brain, with fluid build-up that was likely causing the headaches. A biopsy a few days later gave her a diagnosis of pilocytic astrocytoma, a slow-growing, low-grade brain tumor.
“The last thing any of us expected was a brain tumor,” she says. “I’ve never even known anyone who has had a brain tumor.”
She focused on the positive side of the news — the brain tumor was low-grade — and scheduled a surgery for February to have it removed. But when the tumor grew between the initial biopsy and the tumor resection, her care team turned to a new tool available at Northwestern Medicine hospitals to ensure the initial diagnosis was correct: genomic DNA methylation profiling.
A pattern that reveals an exact diagnosis
For decades, brain tumors have been diagnosed by what pathologists see under a microscope. But recent advances in technology have expanded this toolkit to include DNA methylation profiling.
Methylation is a chemical modification of DNA. Millions of spots on the genome can be chemically modified; in fact, this is part of the process that allows undifferentiated cells to become lung cells or brain cells, for example. The process is binary — each spot on the genome is either methylated or not. Taken alone, the data at any given spot doesn’t mean much. But if you’re able to see the methylation pattern of hundreds of thousands of spots, that creates a pattern that is unique to, for example, certain types of tumors. That means each kind of tumor has its own methylation fingerprint.
It’s like looking at a pointillist painting. It’s only when you step away from the painting that you see the pattern, and that’s what methylation profiling is. It’s an impressionist painting for a tumor.
CRAIG HORBINSKI, MD, PHD
“It’s like looking at a pointillist painting,” says Craig Horbinski, MD, PhD, director of Neuropathology in the Department of Pathology and director of the Pathology Core of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “It’s only when you step away from the painting that you see the pattern, and that’s what methylation profiling is. It’s an impressionist painting of a tumor.”
The technology has only been around since 2017, and this sort of profiling is currently only offered at a few sites around the world. The pathology teams at Northwestern Medicine wanted to bring this service to Illinois. To do so, they started with a library of more than 3,900 brain tumor samples from Germany. Lucas Santana dos Santos, PhD, assistant professor of Pathology and the department’s director of bioinformatics, developed an algorithm that used machine learning to classify tumors according to their methylation profile. He trained the algorithm with the initial dataset (which was no easy feat, considering each tumor has more than 800,000 pieces of genomic data).
“It’s a beautiful application of using artificial intelligence for medicine,” says Santana dos Santos, who works under the direction of Lawrence Jennings, MD, PhD, director of genomic pathology in the Department of Pathology and associate professor of Pathology.
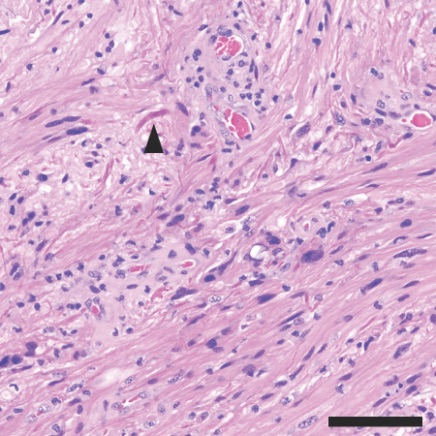
Under the microscope, the pineal tumor was composed of cells with very few mitoses, suggesting a relatively lower- grade tumor. Structures called Rosenthal fibers were present (arrowhead), which also suggested a lower-grade tumor.
When the algorithm was fully trained in fall 2021, the pathology team began profiling central nervous system tumors from biopsies and resections at the Lou and Jean Malnati Brain Tumor Institute of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. When they compare the tumor profile against those found with the machine learning algorithm, they find an exact match for that tumor type.
Since then, the team has profiled more than 1,000 tumor samples — both Northwestern Medicine and its affiliated hospitals, and from outside consults that come in from across the country. Some samples are common types of tumors that clinicians see dozens of times per year. Others are rare. Each one will ultimately help build the library and make the tool even more powerful.
An accurate diagnosis can both give patients peace of mind and also help with treatment. By understanding the exact type of tumor they are dealing with, clinicians can suggest the best course of therapy.
Horbinski estimates methylation profiling confirmed the suspected diagnosis around 80 percent of the time and refined the diagnosis around 15 percent of the time. In 5 percent of the cases, it completely changed the diagnosis.
“As pathologists, we can be faced with uncertainty,” he says. “Is this a primary tumor, or did it spread from somewhere else? Some tumors look worse than they actually are. Pathologists can look at the same sample and diagnose it different ways. Methylation profiling is extremely accurate, and now when it does change the initial diagnosis, often we see that the tumor is not as bad as we thought it was, and patients can survive a lot longer than we initially thought.”
A reason for hope
When methylation profiling confirmed that Fiorito’s tumor was pilocytic astrocytoma — a grade 1 tumor with a good prognosis — she was relieved but knew she faced a long road ahead. After the surgery to remove the tumor, which was located on her pineal gland, she underwent speech and physical therapy at the Shirley Ryan AbilityLab. As a result of the surgery, she suffered from nystagmus (an involuntary, repetitive movement of the eyes), and she had to relearn to walk — a humbling experience for someone used to racing in triathlons and half-marathons.
“I couldn’t walk by myself, and my eyes were very sensitive and blurry, so they told me I needed to walk with a walker, and I told them no, I wasn’t going to do that,” she laughs. Because the tumor couldn’t be completely removed, she also underwent proton radiation, which sapped her energy and caused some of her hair to fall out.
Still, she did eventually begin to run again and even ran a 5K this past fall. Amidst everything, she also got married in September.
“It was a miracle,” she says. “Before the surgery, I was just praying not to die, and somehow through all of that we planned a wedding. It was pretty amazing.”
She has MRIs every three months to monitor the tumor, which remains stable, and is open about sharing the story of her journey. “When people hear they have a brain tumor, it can be scary. I still have a lot of anxiety about it. But maybe somebody will read my story, and it will give them hope that it will get better for them,” she says.
The gold standard for years to come
The pathology team plans to continue to re-train their algorithm with information from the latest samples and is expanding the technology to include sarcomas. Horbinski predicts this could eventually be used for many different types of cancers.
“Methylation is going to be the gold standard for the coming years,” he says. “We hope to continue to build our libraries and make this technology even better, and outside hospitals should consider sending their pathology material to us to validate the diagnosis. We want to ensure that everyone has access to this powerful and important layer of objectivity.”